CAR-X Immunotherapy
1. CAR-X immunotherapy
CAR-X immunotherapy is an emerging field focused on engineering immune cells to express CARs, enabling them to target and eliminate cancer cells. These therapies are classified based on the type of immune cell used (e.g. CAR-T, CAR-NK) and the source of the cells (autologous or allogeneic). The successful clinical application of CAR-T cell therapy, as exemplified by Kymriah, has propelled further research and development in this area.
2. Chimeric antigen receptor ( CAR )
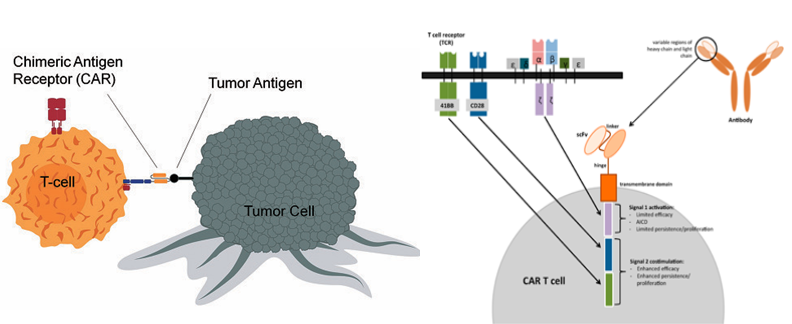
Chimeric antigen receptors (CARs) are synthetic receptors designed to redirect T cells against tumor antigens. They are composed of an antigen-binding domain, a transmembrane domain, and intracellular signaling domains.
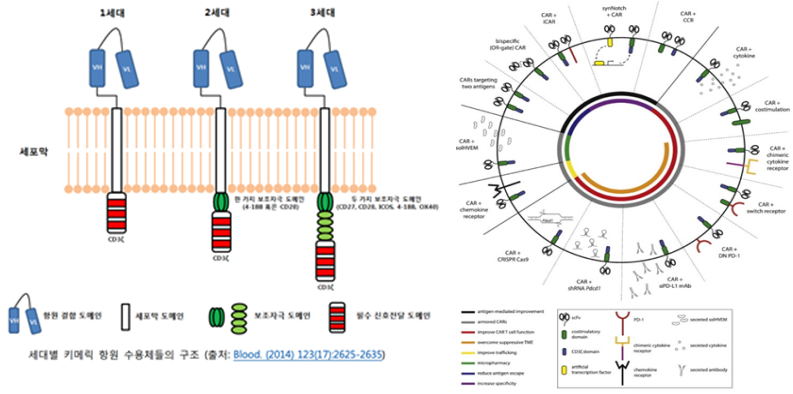
Over generations, from the first to the third, the co-stimulatory domain has been modified to enhance the self-proliferation ability of cells. This allows a small number of injected cells to produce a large number of cells to combat cancer within the body and to persist in the body for a long time.
Beyond these basic structures, various CAR designs are continuously being developed to overcome limitations and enhance efficacy. These include dual-target receptors that can recognize two antigens and receptors that respond to specific stimuli.
3. Research trends of CAR immunotherapy
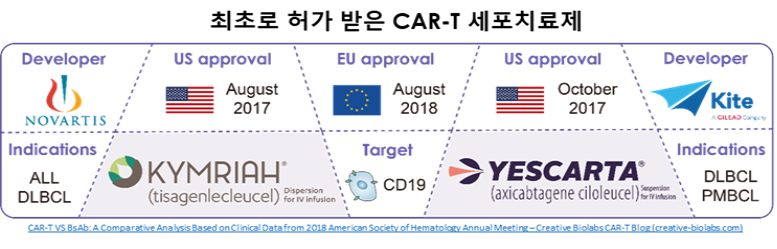
KYMRIAH and YESCARTA are examples of currently approved cell therapies. CAR-X therapies, with their demonstrated efficacy in treating blood cancers, have emerged as a leading candidate for next-generation cancer treatments.
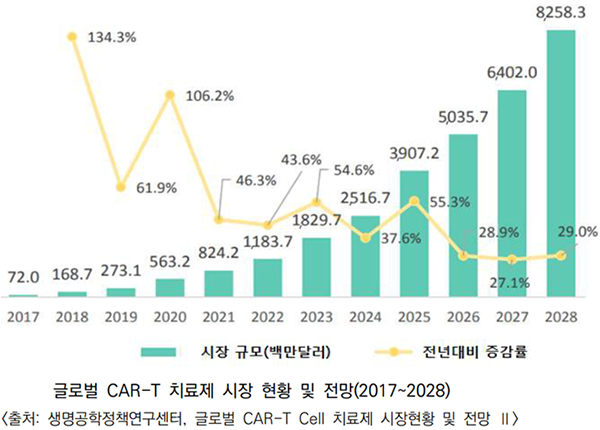
The cell therapy market is projected to be primarily driven by CAR-T therapies. The global CAR-T therapy market is expected to experience significant growth, expanding from $72 million in 2017 to $8.3 billion by 2028, with a CAGR exceeding 53.9%. South Korea has actively pursued research and development in CAR-T cell therapy, and commercialization of these therapies is gaining momentum.
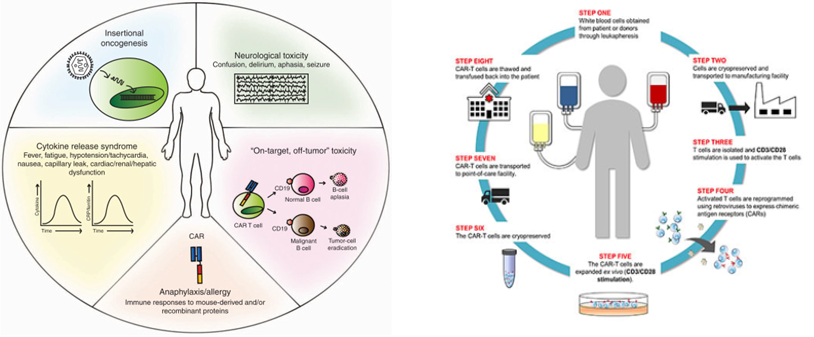
4. Limitations of CAR-T cell therapy
All currently approved CAR T-cell therapies are autologous, meaning they are derived from each patient’s own T cells. This necessitates a multi-step process involving blood collection, T cell isolation, genetic modification, expansion, and selection, making it a personalized approach with a high production cost. Furthermore, the inherent limitations of genetically manipulating immune cells impede the integration of diverse technologies.
While demonstrating remarkable efficacy against hematological malignancies, CAR T-cell therapy currently exhibits limitations. These include its restricted application to primarily blood cancers, the occurrence of adverse events like cytokine release syndrome, and the potential risk of oncogenesis associated with the random integration of the CAR gene using viral vectors. Despite its groundbreaking success, ongoing research is crucial to address these challenges and expand the clinical applicability of this promising therapeutic modality.
5. Current CAR-X immunotherapy research topics in our lab
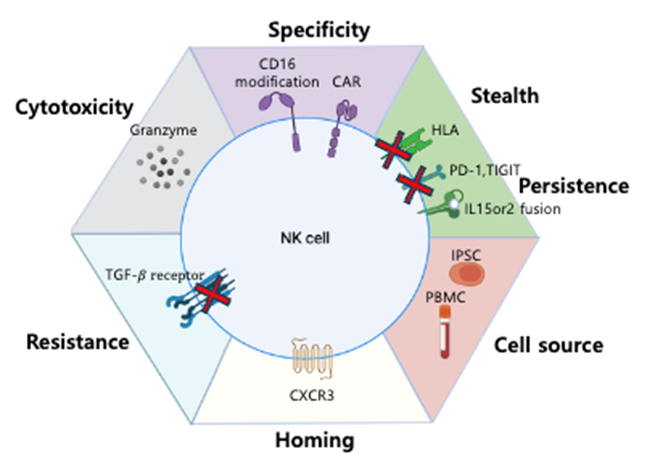
Our research team is actively exploring various strategies to address the limitations of CAR-T cell therapy. To simplify the complex manufacturing process, we are investigating allogeneic cell therapies using an off-the-shelf approach, where a universal product is created from a single donor and can be administered to multiple patients. The cells for CAR-X immunotherapy can be derived from various sources such as peripheral blood mononuclear cells (PBMCs) from healthy adult donors, umbilical cord blood (UCB), and induced pluripotent stem cells (iPSCs). Our laboratory is currently focusing on developing NK cell therapies derived from hematopoietic stem cells from iPSCs and UCB. For iPSC-derived NK cells, we have developed a site-specific gene integration platform to minimize the risk of random mutations by enabling multiple rounds of gene editing and selection. For UCB-derived NK cell therapies, we are conducting research to address the limitations of T cells, such as high adverse effects, by differentiating hematopoietic stem cells from iPSCs into NK cells and developing methods to expand NK cells. Based on these foundational studies, we are employing the strategy illustrated in the right-hand figure to enhance the efficacy of NK cell therapies.