Liquid Biopsy (LBx)
1. Definition and value of LBx
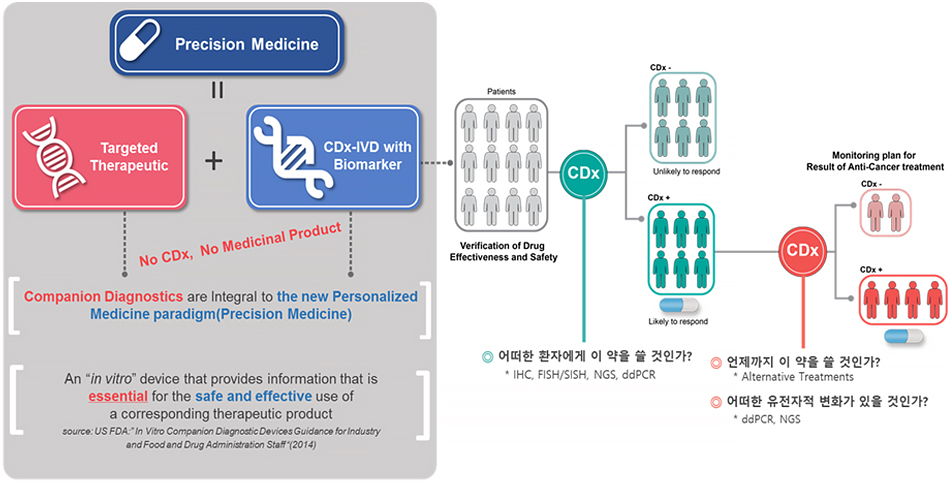
The primary value of liquid biopsy lies in its ability to reduce adverse drug reactions by preventing the administration of ineffective medications to non-responsive patients, thereby increasing the cost-effectiveness of treatment and ensuring patients receive the most appropriate therapy from the outset.
For instance, while Herceptin is an effective targeted therapy, 5-30% of patients undergoing chemotherapy and Herceptin treatment experience cardiotoxicity. If a HercepTest liquid biopsy test indicates a patient is likely to benefit from the drug, they can make an informed decision about accepting the risk of this side effect. However, if Herceptin were administered indiscriminately, many patients would undergo ineffective treatment and suffer from cardiotoxicity. Additionally, targeted therapies currently available on the market are approximately five times more expensive than conventional chemotherapy drugs, leading to significant financial burdens on both patients and healthcare systems, a phenomenon termed “financial toxicity” in the United States. By using liquid biopsy to selectively administer these high-cost targeted therapies to patients who are most likely to benefit, we can alleviate the financial burden on patients and healthcare systems and improve the cost-effectiveness of targeted therapy. Moreover, the opportunity to provide patients with the most suitable treatment options is a value that cannot be overlooked.
2. Current status of liquid biopsy research and development
2-1) Current status in Korea
Most of the cancer gene mutation detection kits in Korea have obtained MFDS (Ministry of Food and Drug Safety) IVD class 2 & 3 approval as amplification reagents. Some products have been recognized as new technologies through new medical technology assessments and have been assigned insurance rates by the Health Insurance Review and Assessment Service, allowing them to be sold and used for diagnosis.
However, in Korea, the development and approval of pharmaceuticals and liquid biopsy devices are still conducted separately (pharmaceuticals: Pharmaceutical Affairs Act, Liquid biopsy devices: Medical Devices Act). To meet the market demands and achieve successful new drug development in a global market environment, pharmaceutical companies and diagnostic kit developers are planning and implementing joint development, leading to some side effects.
2-2) Global Status
Driven by the increasing demand for targeted therapies to reduce drug development costs for pharmaceutical companies, decrease adverse effects, and improve quality of life, the liquid biopsy market has been growing at an annual rate of 18% from 2013 to 2019. This market is primarily dominated by cancer gene mutation detection kits for breast, lung, colorectal, gastric, and melanoma cancers. Common techniques used in these kits include immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), chromogenic in situ hybridization (CISH), and quantitative polymerase chain reaction (q-PCR). Recently, diagnostic kits utilizing next-generation sequencing (NGS) have also been developed. As of July 2019, the FDA has approved a total of 39 in vitro liquid biopsy devices. With over 85 liquid biopsy tests currently on the market and a growing pipeline, this sector is experiencing rapid growth. Large pharmaceutical companies are increasingly collaborating with liquid biopsy to co-develop liquid biopsy (LBx), leveraging the latter’s technological expertise and know-how.
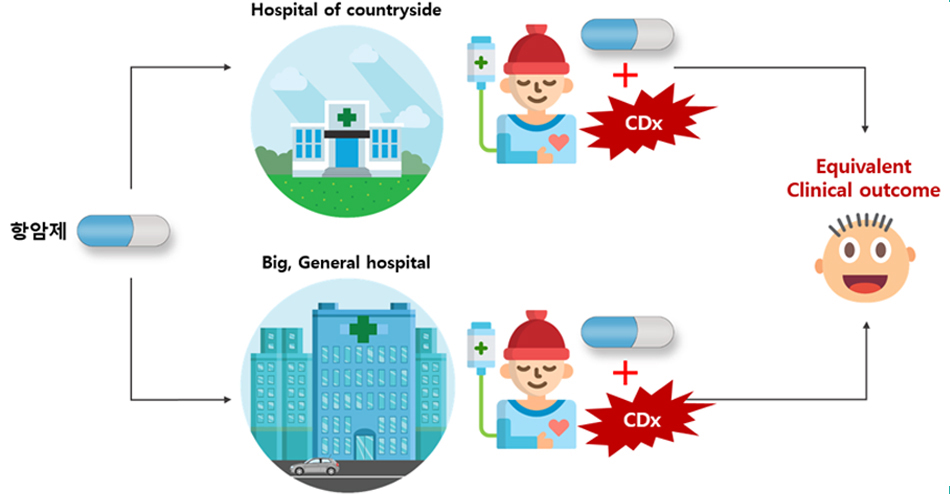
3. The ultimate goal of LBx
The ultimate goal of liquid biopsy is to enable the selection of cancer treatment candidates in hospitals globally based on liquid biopsy, with the aim of achieving consistent clinical outcomes across all patients.
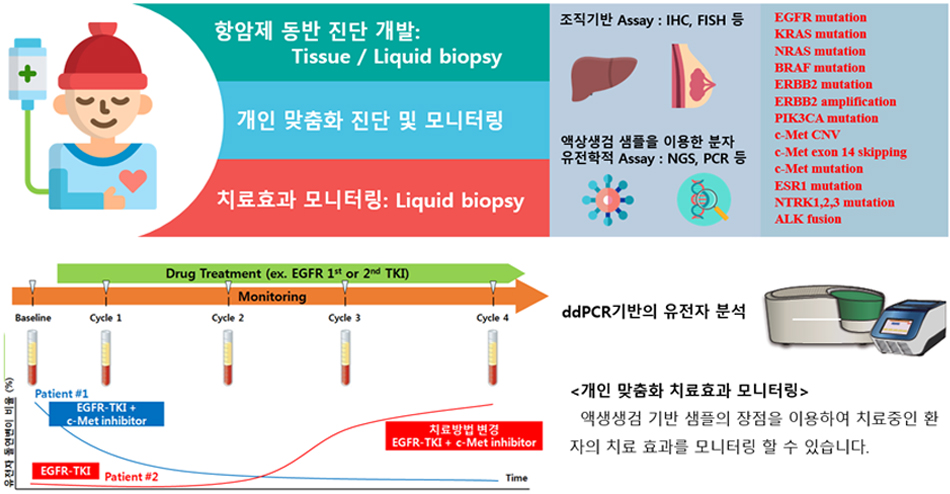
4. Current biomarker discovery research topics in our lab
4-1) Liquid biopsy research for cancer
Targeted cancer therapies, exhibiting superior efficacy with reduced adverse effects, operate by targeting specific molecular alterations within cancer cells. To optimize treatment outcomes, it is imperative to pre-select patients based on their genetic profiles and drug target expression. Liquid biopsy, by providing a robust clinical rationale for treatment decisions, can enhance treatment efficacy and reduce healthcare costs associated with the inappropriate use of targeted therapies. Our laboratory is dedicated to the development of diagnostic kits that leverage biomarkers for patient stratification.
4-2) Circulating tumor cell research & single cell genomics
Conventional biomarker diagnostic methods, including IHC, FISH, and CGH arrays, while valuable, are constrained by their reliance on tissue samples, limiting their ability to capture the dynamic nature of tumor heterogeneity. Liquid biopsy, an emerging approach that analyzes circulating tumor cells and cell-free nucleic acids in blood, offers a non-invasive and more comprehensive assessment of the tumor microenvironment. By employing sensitive techniques such as NGS and ddPCR, liquid biopsy enables real-time monitoring of tumor evolution and facilitates personalized treatment strategies, thereby improving patient outcomes.
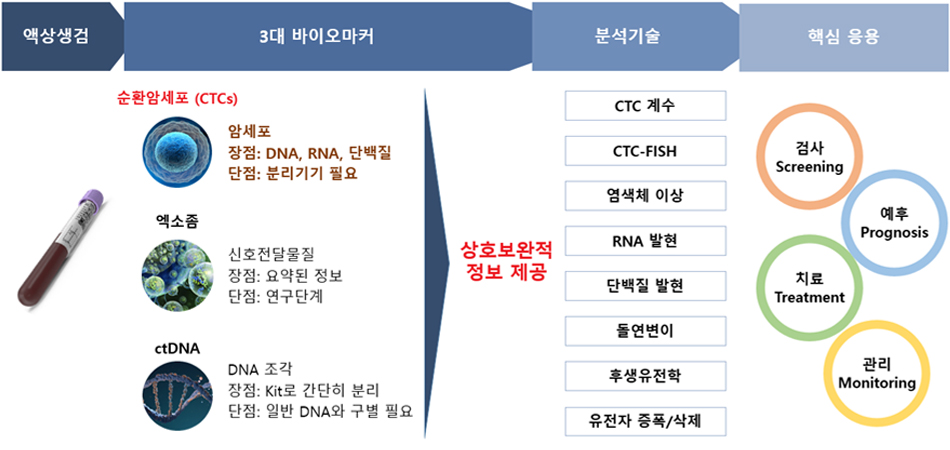
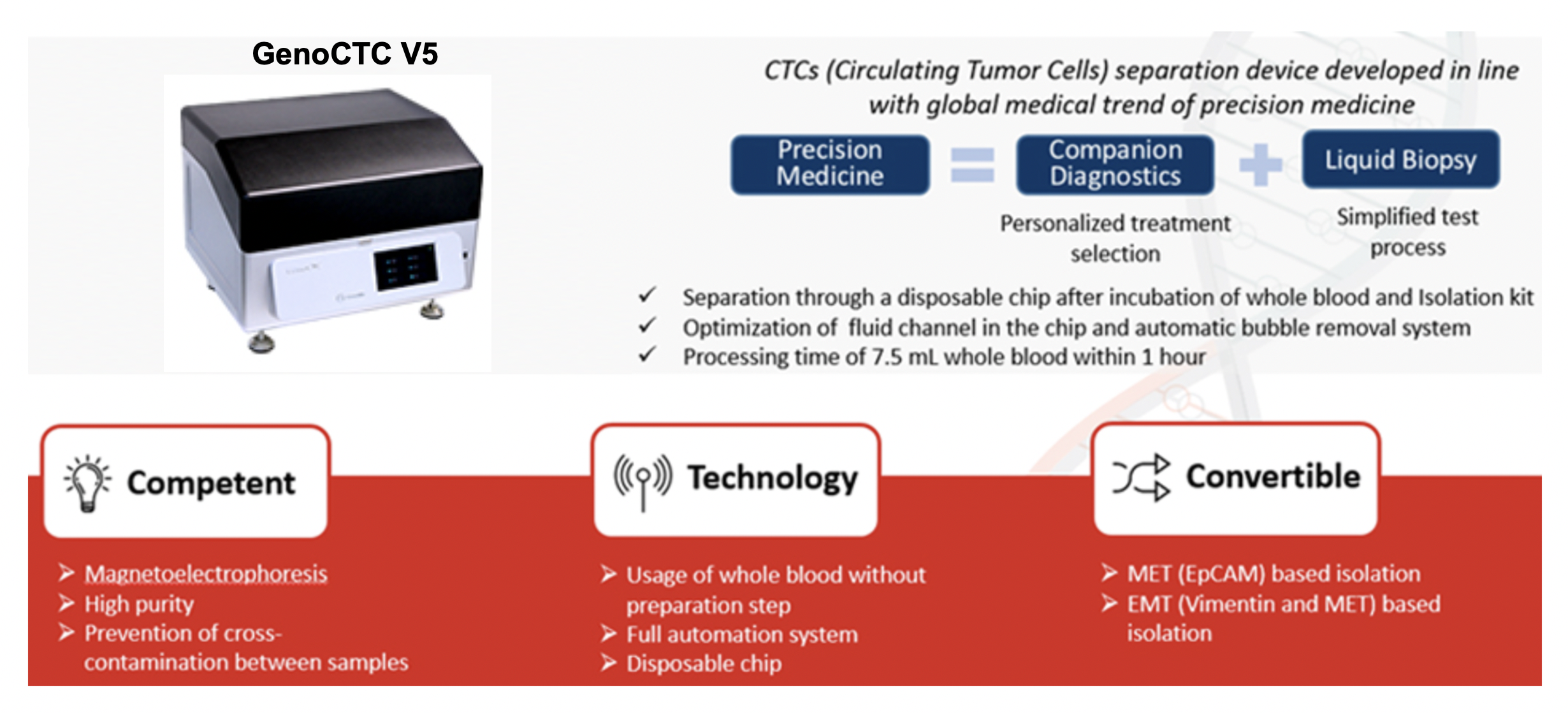
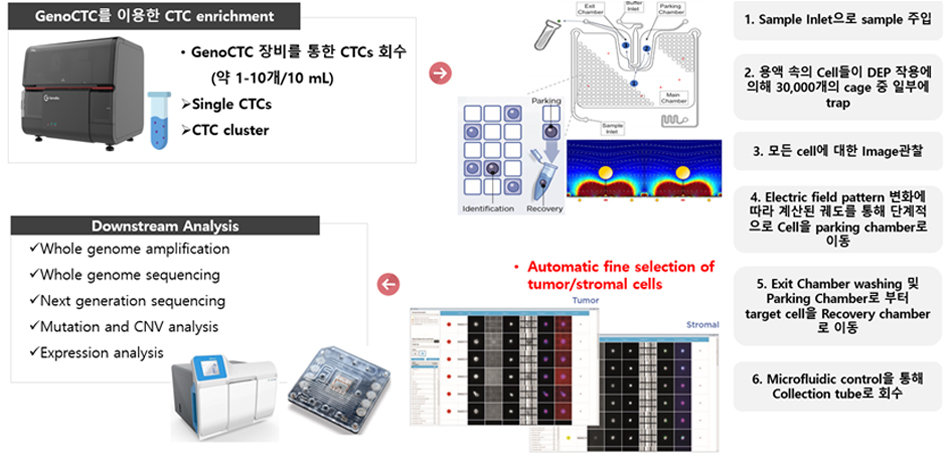
Our research aims to investigate cancer characteristics and develop liquid biopsy applications through the use of GenoCTC, a state-of-the-art platform for isolating highly purified circulating tumor cells.
Tumor heterogeneity, characterized by the coexistence of genetically distinct tumor cell subclones, often spatially segregated, results in phenotypic diversity within tumors. This intratumoral heterogeneity can lead to the emergence of drug-resistant subclones, contributing to therapeutic resistance and relapse. Our laboratory is investigating the mechanisms underlying tumor heterogeneity at the single-cell level. Specifically, we are utilizing GenoCTC to isolate CTCs for subsequent immunohistochemical staining and whole genome amplification to characterize protein expression and genetic alterations, respectively.